Developments in Rhodium Catalyzed C H insertion of Donor/Donor Carbenes
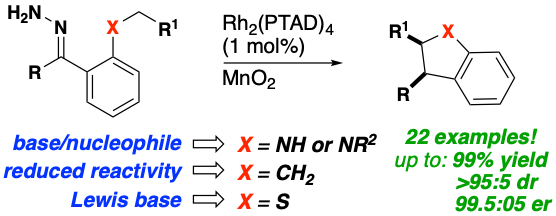
In a recent publication (Souza, Squitieri et al, Enantioselective Synthesis of Indolines, Benzodihydrothiophenes, and Indanes by C−H Insertion of Donor/Donor Carbenes 2018) the Shaw Lab employs a single catalyst/oxidant system to enable the asymmetric syntheses of indolines, benzodihydrothiophenes, and indanes by C−H insertion of donor/donor carbenes. This methodology enables the rapid construction of densely substituted five‐membered rings that form the core of many drug targets and natural products. Furthermore, oxidation of hydrazones to the corresponding diazo compounds proceeds in situ, enabling a relatively facile one‐ or two‐pot protocol in which isolation of potentially explosive diazo alkanes is avoided. This methodology was applied to a variety of substrates in high yield, diastereomeric, and enantiomeric ratios and to the first enantioselective total synthesis of a patented indane estrogen receptor agonist with anti‐cancer activity.
https://onlinelibrary.wiley.com/doi/full/10.1002/ange.201809344
