Zeolites exhibit flexibility during H2O and CO2 absorption
Thermodynamic and structural evidence of flexibility during H2O and CO2 absorption in transition metal ion exchanged zeolite A (LTA topology)
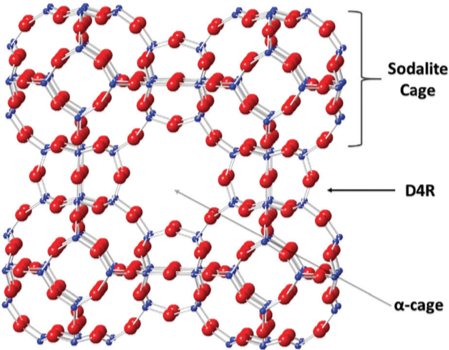
Scientific Achievement
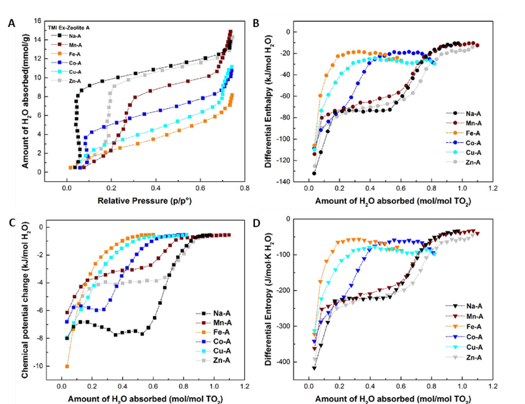
Gas absorption calorimetry was customized to determine the energetics of H2O and CO2 absorption on zeolite A as a function of loading from near zero coverage to saturation, Results provide clear thermodynamic evidence of structural flexibility induced by guest molecules and identify different strengths of interactions at various positions (cages and sites). XRD and FTIR during hydration showed rapid water uptake and structural changes leading to complete rehydration within about an hour at ambient temperature.
Significance and Impact
Framework flexibility during water absorption leads to a phase transition, similar to gate opening, and to a two-phase region between the hydrated and dehydrated forms, both of which, rather than being stoichiometric, have variable water content. It is likely that the absorption capacity can be more closely controlled by further modification of the hydrophilicity and gating cations. The flexibility of zeolite A can be tuned for selective absorption in processes operating in the presence of water or gas mixtures.
Research Details
The differential enthalpy of water absorption by Zn-A and Mn-A are most exothermic, -125.28 ± 4.82 and –115.30 ± 2.56 kJ mol-1 . These two zeolites also show moderately good capture ability for CO2 with zero-coverage enthalpies of -55.59 ± 2.48 and -44.07 ± 1.53 kJ mol-1.
X. Guo and A. Navrotsky, Phys. Chem. Chem. Phys., 20, 3970-3978, 2018, Micropor. Mesopor. Mat., 268, 197–201, 2018
