Ultrafast spectroscopy reveals photoinduced charge transfer dynamics in iron-sulfur proteins
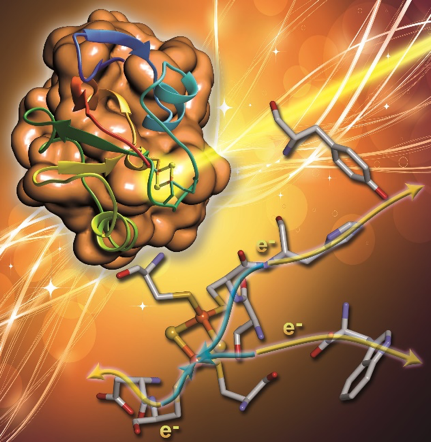
A combined effort between the Larsen, Cramer and Goodin labs characterized direct charge-transfer photodynamics in four iron-sulfur (FeS) proteins with femtosecond transient absorption spectroscopy. The labs identified multiple ligand-to-metal charge transfer populations were found to be induced by laser excitation that evolve into low lying charge-transfer states, which is mediated by the density of states and reorganization energy. Two long-lived states were identified in the 2Fe-2S and 4Fe-4S clusters and are attributed to a long-range “external” charge transfer, which suggests the potential existence of a photo-induced long-range electron transfer pathway in FeS proteins, suggesting that they are the optimal candidates as external photosensitizers for photo-induced chemical reactions such as solar hydrogen production.
More information at http://pubs.acs.org/doi/abs/10.1021/acs.biochem.7b01159
