Thermodynamics of H₂O and CO₂ Absorption and Guest-induced Phase Transitions in Zeolite RHO
Scientific Achievement
The zeolite RHO structure is unusually flexible in response to guest molecule occupancy. Gas absorption calorimetry determined the energetics of H₂O and CO₂ interaction with the framework as a function of loading from near zero coverage to saturation for several cation exchanged zeolite RHO materials. Framework flexibility and an associated “gate opening” phase transition from the dehydrated acentric form to the hydrated centric form during water absorption are clearly detected and quantified. The interactions with CO₂ are weaker than with H₂O.
Significance and Impact
All the compositions studied show substantial framework flexibility. Lithium may be the best performing ion to induce flexibility and may provide a promising avenue to activate “gate opening” and achieve better gas storage capacity and catalytic properties due to its more dynamic response to guest molecules.
Research Details
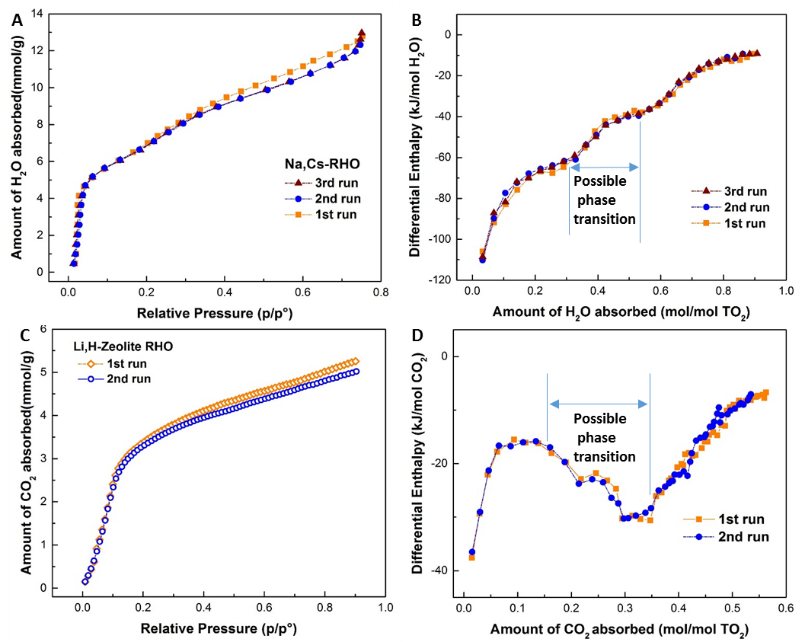
Gas absorption calorimetry was performed on the dehydrated Zeolite RHO samples at room temperature. The differential enthalpy of absorption by Cd,Cs-RHO shows most exothermic values with -163.74 ± 4.63 and –46.45 ± 0.44 kJ mol-1 in H₂O and CO₂ sorption respectively followed by Na,Cs-RHO with 108.27 ± 2.46 and –40.68 ± 0.81 kJ mol-1. The Li,H-RHO exhibits highest water uptake and the capture of CO₂ with zero-coverage enthalpy of -128.70 ± 2.99 and -37.13 ± 0.65 kJ mol-1 respectively. The “gate opening” phase transition activated by CO₂ sorption was observed for Li,H-RHO at a pressure as low as 0.1 bar.
J. Phys. Chem. C, 2018, DOI:10.1021/acs.jpcc.8b06070