Streamlined chemoenzymatic total synthesis of prioritized ganglioside cancer antigens
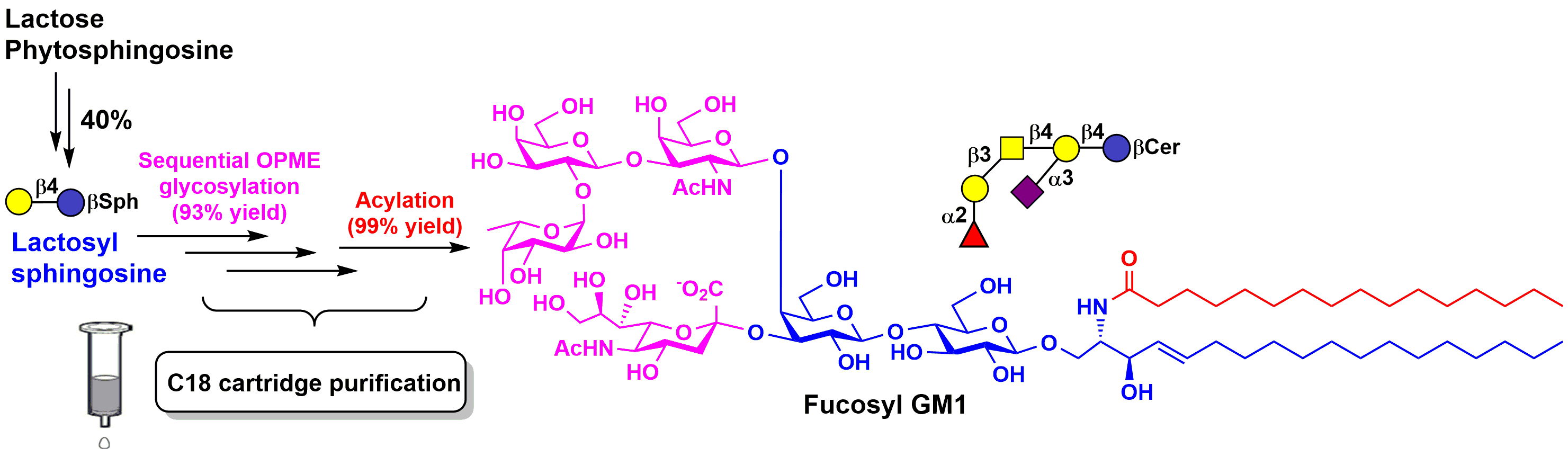
Ganglioside antigens expressed by human cancer cells and are attractive but challenging synthetic targets. In a recent publication, (Chen and coworkers, Organic & Biomolecular Chemistry, 2018) describes a highly efficient streamlined chemoenzymatic process for total synthesis of synthetically challenging complex gangliosides. Sequential one-pot multienzyme (OPME) enzymatic glycosylation of chemically synthesized lactosyl sphingosine, followed by high-yield acylation, and combined with facile C18-cartridge purification scheme, successfully led to the production of negatively charged complex gangliosides. Four prioritized ganglioside cancer antigens including GM3, fucosyl GM1, GD3, and GD2 are successfully obtained in high yields. Among which, the first total synthesis of fucosyl GM1, a hexasaccharyl ceramide, was achieved in a total yield of 37% from commercially available inexpensive lactose and phytosphingosine. This synthetic and purification strategy is readily applicable to other challenging complex glycosphingolipid targets.
More information at http://pubs.rsc.org/en/Content/ArticleLanding/2018/OB/C8OB01087K#!divAbstract
