A recipe for docking carbocations in enzyme active sites
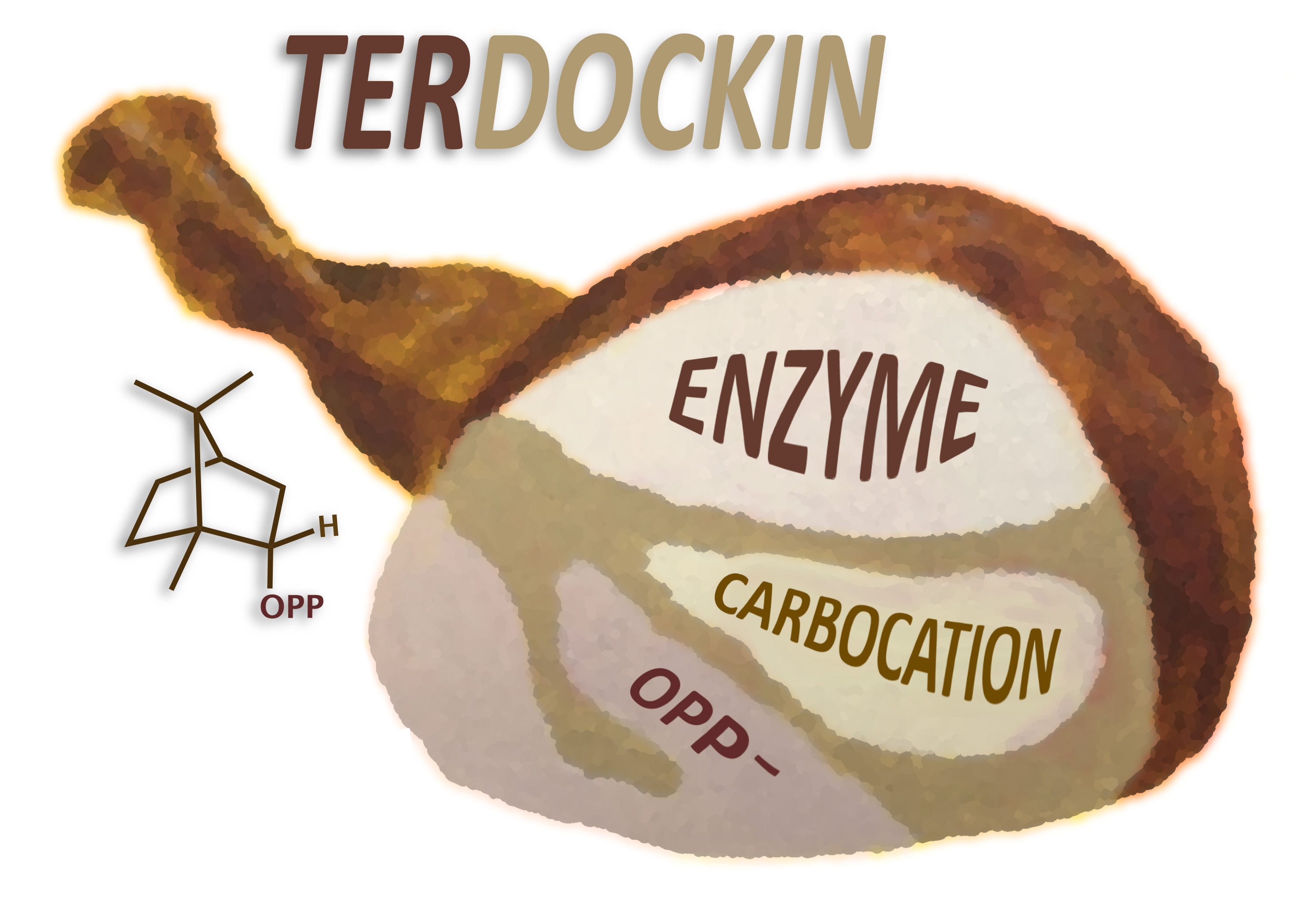
Terpene synthases are enzymes that convert acyclic substrates to complex, polycyclic terpene natural products via carbocation intermediates. Predicting mechanistically relevant binding modes for carbocations in terpene synthase active sites is a well-recognized and daunting challenge, due, in large part, to the fact that both the carbocations and active site groups that surround them are largely hydrophobic. In a recent publication (O’Brien and co-workers, ACS Catalysis 2018, DOI: 10.1021/acscatal.8b00342) the Siegel and Tantillo labs describe an effective method for addressing this long-standing challenge. Binding modes predicted using their method, called TerDockin (for terpene docking), set the stage for subsequent simulations and enzyme design efforts.
