A Radical Relay Mechanism for HydG from quantum chemistry calculations
Wang and Britt lab research published in Biochemistry
A Radical Relay Mechanism for HydG from quantum chemistry calculations
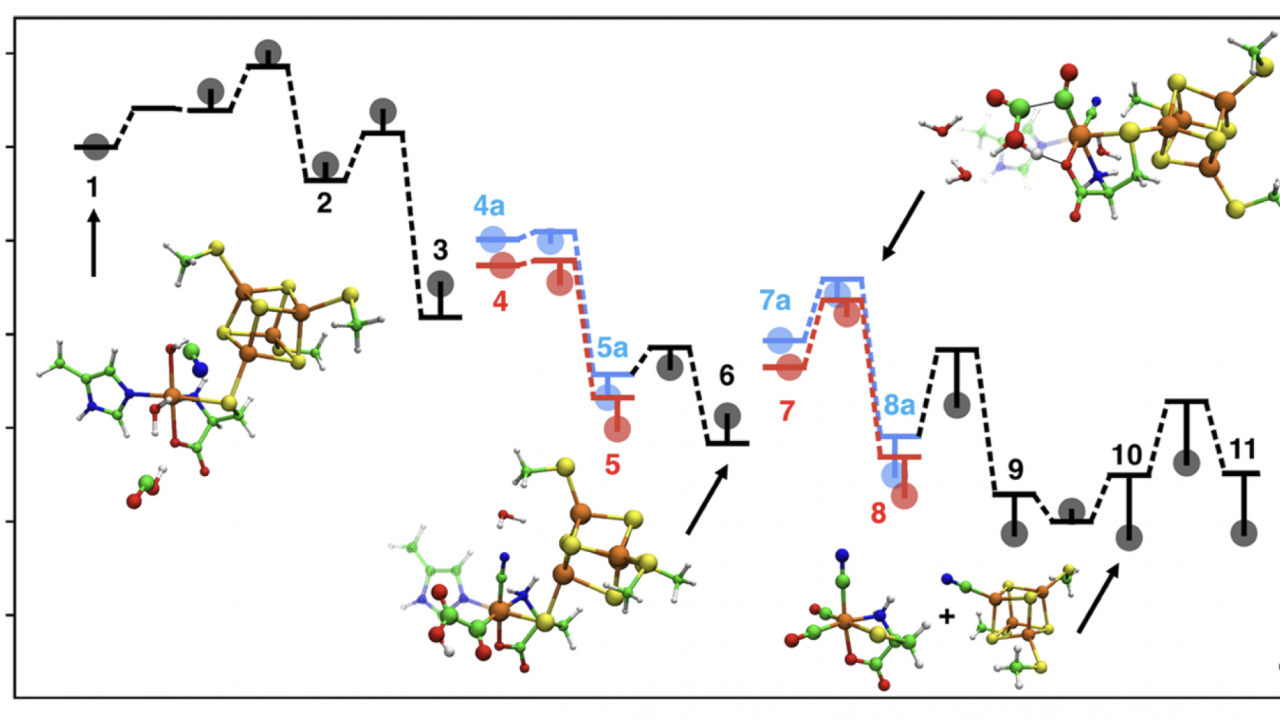
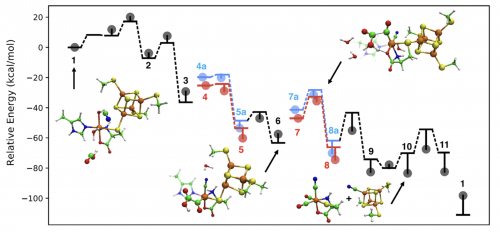
In a study recently published in Biochemistry, the Wang and Britt labs collaborated to elucidate the catalytic mechanism of the HydG maturase enzyme, which plays a key role in the biosynthesis of the active site of [FeFe] hydrogenase, an enzyme that can reduce protons to H₂. This theoretical study addresses several open mechanistic questions that have been difficult to access experimentally regarding this radical SAM (S-adenosyl-methionine) enzyme. The main results include a radical relay mechanism that describes how a dehydroglycine intermediate, formed by radical-initiated decomposition of a tyrosine substrate, is further decomposed into diatomic CO and CN ligands; and a redox-mediated catalytic cycle that assembles two equivalents of these ligands into the Fe(CO)₂(CN)cysteine “synthon” product for further processing by the next enzyme in the assembly line, HydE. This study sheds light on the synthesis and maintenance of enzyme catalysts for renewable energy conversion, and bolsters the ongoing experimental studies in the Britt Lab to fully map the biosynthesis pathway of the [FeFe] hydrogenase active site.
More details at https://pubs.acs.org/doi/10.1021/acs.biochem.1c00379 .
