Hidden protein structures key to understanding neurodegenerative disease
The latest publication from the Murray Laboratory describes new structural characteristics of the transcription factor protein TDP-43 and provides insight into the pathology of Amyotrophic Lateral Sclerosis and Frontal Temporal Dementia. The work has been honored by the American Chemical Society as an Editor’s Choice Article because it embodies the society’s goal of improving the human experience using the power of chemistry.
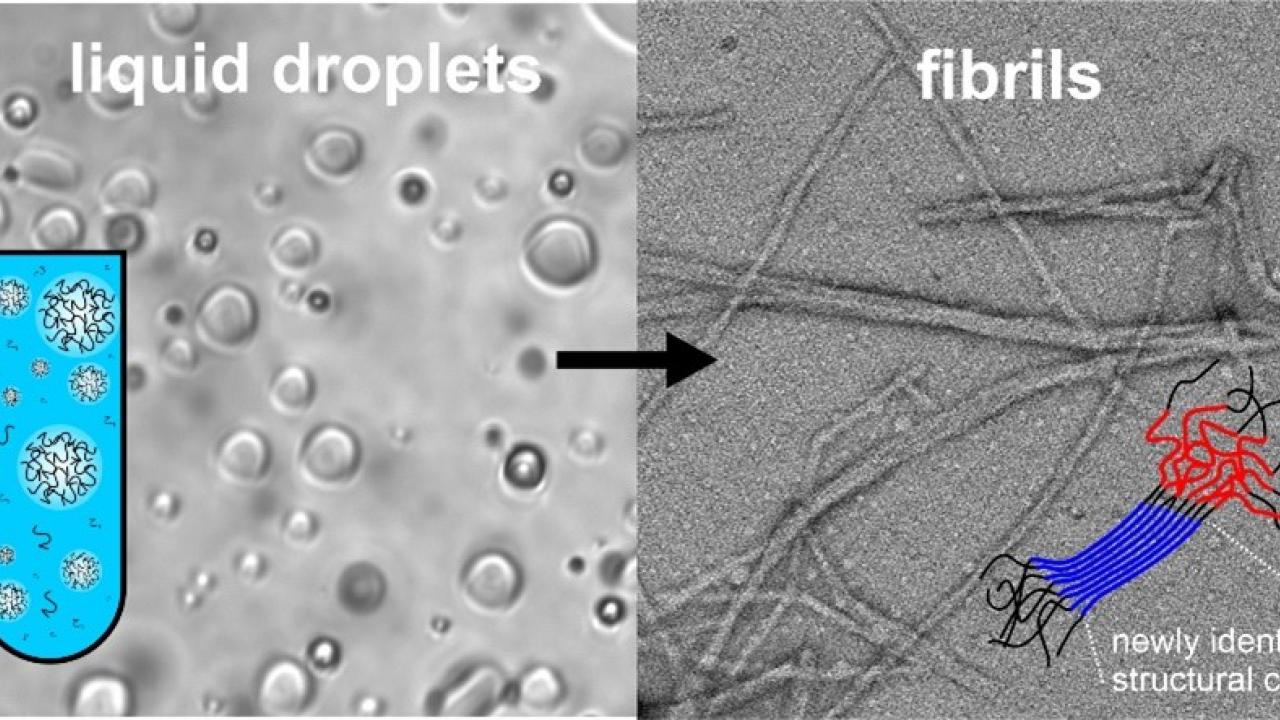
The TDP-43 protein undergoes a series of structural transitions during disease progression. A key and poorly understood step in this process is the conversion from a functional liquid droplet state, analogous to oil droplets in water, to a fibril state associated with nerve cell death. New equipment recently installed at the UC Davis Nuclear Magnetic Resonance Core Facility enabled the research team to identify the parts of the TDP-43 transcription factor protein that form stable structure during the transition from the liquid droplet to fibril state. In a surprising turn of events, the structured region is not the region previously thought to be most important for disease pathology. The research sets the stage for new and more effective treatments of neurodegenerative diseases by providing a more complete understanding of the chemical processes underlying them.
The publisher has made the article freely available for six months online due to its broad public interest. After twelve months the article will be made open access as part of the University of California’s effort to make all research publicly available.
This article was highlighted as a Spotlight in the journal. Read more about it here.
