Fast Proton Transfer and Hydrogen Evolution Mediated by [Co₁₃C₂(CO)₂₄]⁴⁻
In a recent publication (Berben and coworkers, Journal of American Chemical Society, 2020) the Berben Lab brings to light an approach for enhancing rates of hydrogen evolution using a metal-metal bonded molecular catalyst. Experiments demonstrate that [Co₁₃C₂(CO)₂₄]⁴⁻, containing multiple metal-metal bonds boosts the rate of hydrogen evolution compared to single-site metal complexes. The fast catalytic rate is attributed to the presence of multiple binding sites for proton transfer (PT) reactions on the metal-metal bonds: this statistically enhances rates of PT just as H-bonding enhances PT in enzyme active sites and multiple binding sites enhance PT on heterogeneous catalysts. The ability to precisely characterize molecular catalysts at the atomic level enabled thorough characterization of this system. Insight into the mechanisms for fuel-forming reactions that occur at materials with delocalized electronic structures enables improvements in future catalyst design across molecular, nanomaterial and heterogeneous electrocatalyst systems.
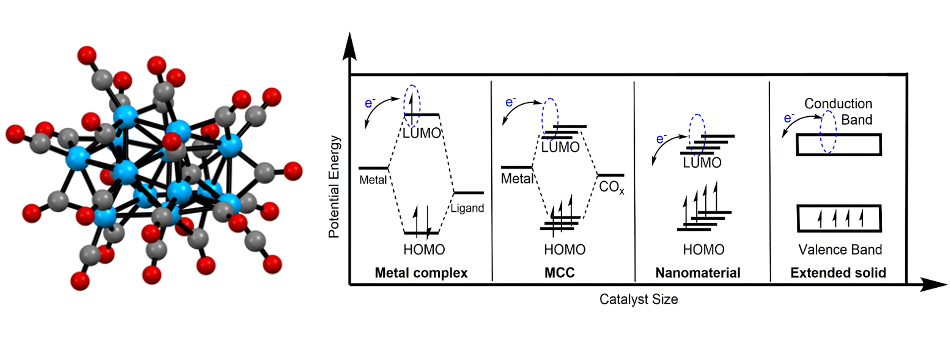
(Left) Hydrogen evolution catalyst: [Co₁₃C₂(CO)₂₄]⁴⁻. Blue, grey and red balls represent Co, C, and O atoms, respectively. (Right) Schematic showing the relative spacings of orbitals in single-site metal complexes, metal carbonyl clusters (MCC), nanomaterials and extended solids; [Co₁₃C₂(CO)₂₄]⁴⁻ is an MCC.
More information available at: https://pubs.acs.org/doi/10.1021/jacs.0c04034
