Electrochemical Reduction of N₂ to NH₃ at Low Potential by a Molecular Aluminum Complex
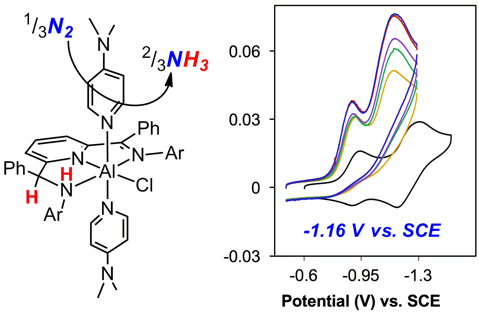
Electrochemical generation of ammonia (NH₃) from nitrogen (N₂) using renewable electricity is a desirable alternative to current NH₃ production methods, which consume roughly 1 % of the world's total energy use. In a recent publication (Berben and coworkers, Chem. Eur. J., 2018) the Berben Lab presents an approach to the electrochemical reduction of N₂ into NH3 using a coordination complex of aluminum(III), which facilitates NH₃ production at a low reduction potential. EPR spectroscopic characterization (collaboration with the Britt Lab) of a reduced intermediate and investigations of product inhibition are presented to provide mechanistic insights into this process.
This article was featured in ChemistryViews, the magazine of ChemPubSoc Europe.
More information at https://onlinelibrary.wiley.com/doi/full/10.1002/chem.201804454
