The biosynthetic origins of [FeFe] hydrogenase active site unraveled
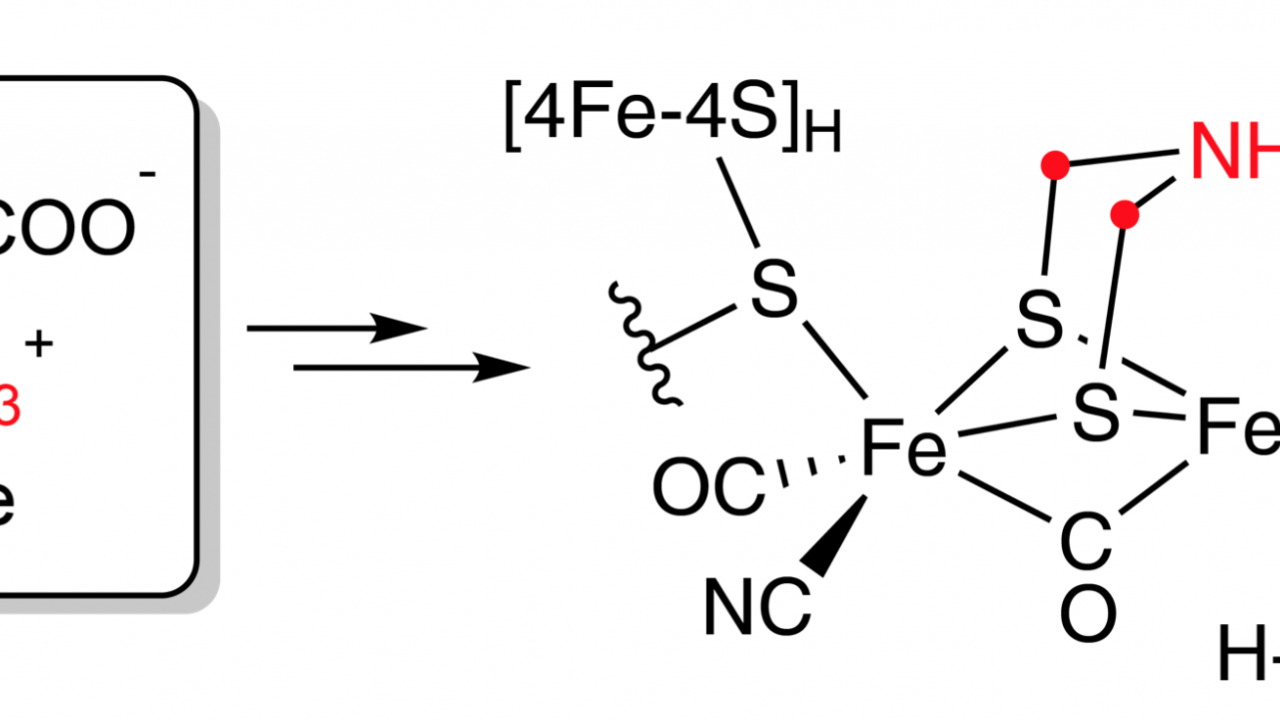
Hydrogenases are nature’s machines to metabolize H₂. The [FeFe] hydrogenase subtype catalyzes the rapid turnover at its six-iron active site cofactor, the H-cluster, which contains CO, cyanide and a unique azadithiolate (adt) ligand essential for catalysis. In a recent publication (Rao and coworkers, Chemical Science, 2019) the Britt lab identified the biosynthetic precursor of the adt ligand. By using a cell-free hydrogenase maturation approach and pulse electronic paramagnetic resonance spectroscopy, the Britt lab discovered that the adt ligand is derived from the amino acid serine. With this finding the biosynthetic origins of the entire H-cluster is now unraveled.
More information at:
https://pubs.rsc.org/en/content/articlelanding/2020/sc/c9sc05900h#!divAbstract
