An Aminoimidazole Radical Intermediate in the Anaerobic Biosynthesis of the 5,6-dimethylbenzimidazole Ligand to Vitamin B12
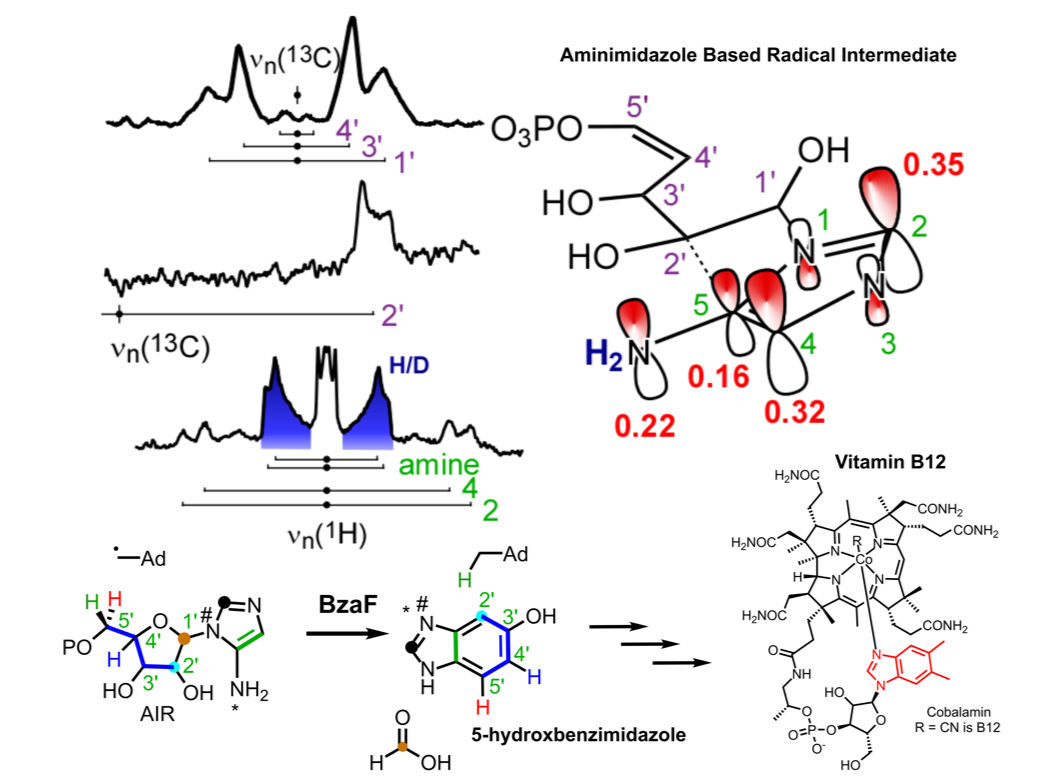
In a recent publication (Gagnon, et. al., Journal of the American Chemical Society, 2008, DOI: 10.1021/jacs.8b05686) the Britt lab utilized freeze quench techniques to trap a radical intermediate in the biosynthesis of the lower ligand to Vitamin B12 by the radical S-adenosyl-L-methionine (SAM) enzyme BzaF. The trapped radical was characterized with advanced electron paramagnetic resonance (EPR) techniques in conjunction with density functional theory (DFT) and numerical simulations to identify an aminoimidazole radical intermediate and propose a structure for the radical intermediate. The proposed radical intermediate structure lead to a rewriting of the proposed mechanism for the dramatic rearrangement of the substrate, aminoimidazole ribotide (AIR), to the product 5-hydroxybenzimidazole (5-HBI). These results provide new insight into how the radical SAM enzyme class carries out its life sustaining chemistry.
More information at https://pubs.acs.org/doi/10.1021/jacs.8b05686
