Ultrafast dynamics of vibrational energy transfer in water
Donadio lab research published in Nature
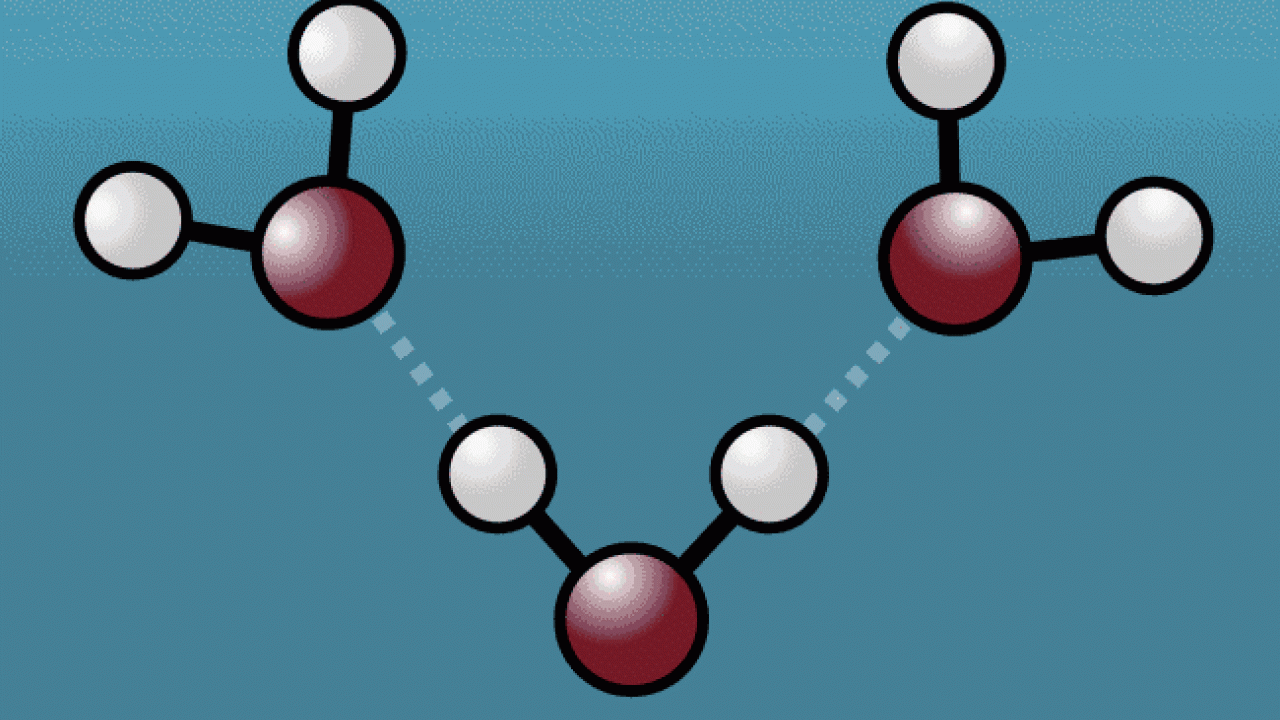
Water is the most abundant yet least understood liquid in nature. A recent collaborative study published in Nature sheds light on the ultrafast process of vibrational energy transfer in water molecules. “Hydrogen bonding, the molecular building block that gives water its special properties, is still not fully understood,” said Davide Donadio, professor of chemistry at UC Davis and a corresponding author on the paper. “This work combines unprecedented experiments and beyond state-of-the-art nonequilibrium molecular simulations to unravel the relaxation dynamics of hydrogen bonds when the intramolecular OH bond is excited by an intense infrared laser.”
The experiments and simulations revealed that as an excited water molecule starts to vibrate, its hydrogen atom tugs oxygen atoms from neighboring water molecules closer before pushing them away with its newfound strength, expanding the space between the molecules. Results from the simulations also confirmed the importance of nuclear quantum effects in hydrogen bonding.
The researchers hope to use this method to gain more insight into the quantum nature of hydrogen bonds and the role they play in water’s strange properties, as well as the key role these properties play in many chemical and biological processes.
Further information:
Scientists Capture ‘Quantum Tug’ Between Water Molecules - Egghead (ucdavis.edu)
Getting beneath the surface of water (acs.org)
Direct observation of ultrafast hydrogen bond strengthening in liquid water | Nature (Original Article)
