David Lab Publishes Work Elucidating a Key Structure-Activity Relationship for the Recognition of Mispairs
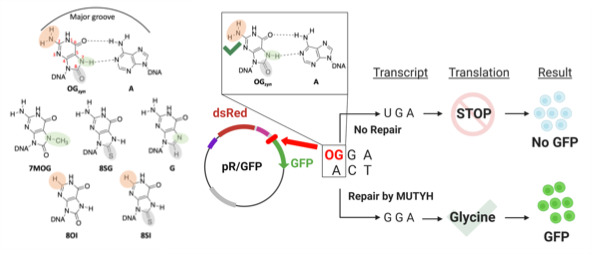
The David Lab recently published an article in ACS Central Science elucidating a key structure-activity relationship for the recognition of mispairs between adenine and the oxidatively damaged 8-oxo-7,8-dihydroguanine (OG) by human DNA repair glycosylase, MUTYH. By evaluating the activity of MUTYH with series of synthetic DNA substrates both in vitro and in cells revealed that MUTYH, unlike the bacterial enzyme, relies almost exclusively on the unique major groove position of the 2-amino group in OGsyn:Aanti mismatches to locate and repair DNA lesions. These findings shed light on how cancer-associated MUTYH variants with deficiencies in the OG detection domain can be severely detrimental to DNA repair. Further, the identification of this key recognition site offers a promising strategy for developing small molecule MUTYH inhibitors for clinical applications.
Access this article at https://pubs.acs.org/doi/10.1021/acscentsci.3c00784