Hydrogenase Catalysis – Collaboration Between Metals and Protein
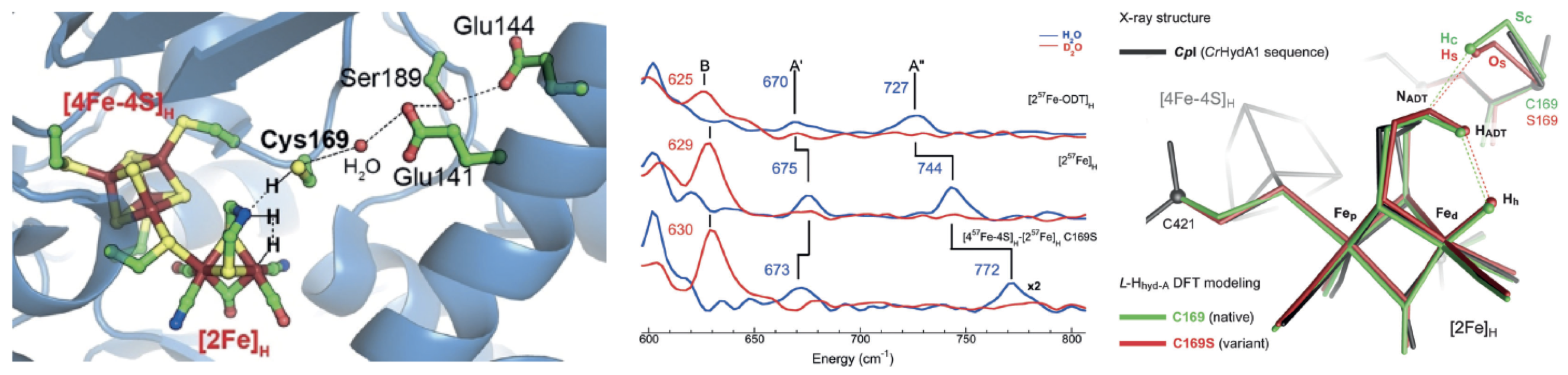
In a recent publication in Angewandte Chemie, the Cramer group and collaborators use a synchrotron technique, NRVS, to probe the coupling between the hydrogenase H-cluster and its neighboring proton transfer chain. NRVS is a vibrational spectroscopy that is only sensitive to the motions of the resonant nucleus, in this case 57Fe vibrations. The team observed a progressive shift of 45 cm-1 in the frequency of an Fe-H bend mode for three H2ase samples: (a) a variant with O replacing N in the aminodithiolate (ADT) bridge, (b) the wild-type enzyme, and (c) a variant with serine O replacing cysteine S at the end of the protein transfer chain. DFT calculations explain the shifts as evidence for coupling of motions between Fe-H, ADT, and the S-H or O-H in the chain. Such coupling may be important for the catalytic mechanism.
More information at DOI: 10.1002/anie.201805144
