Catalyzing Enantioselective Si–H Insertions for Silicon-centered Chirality
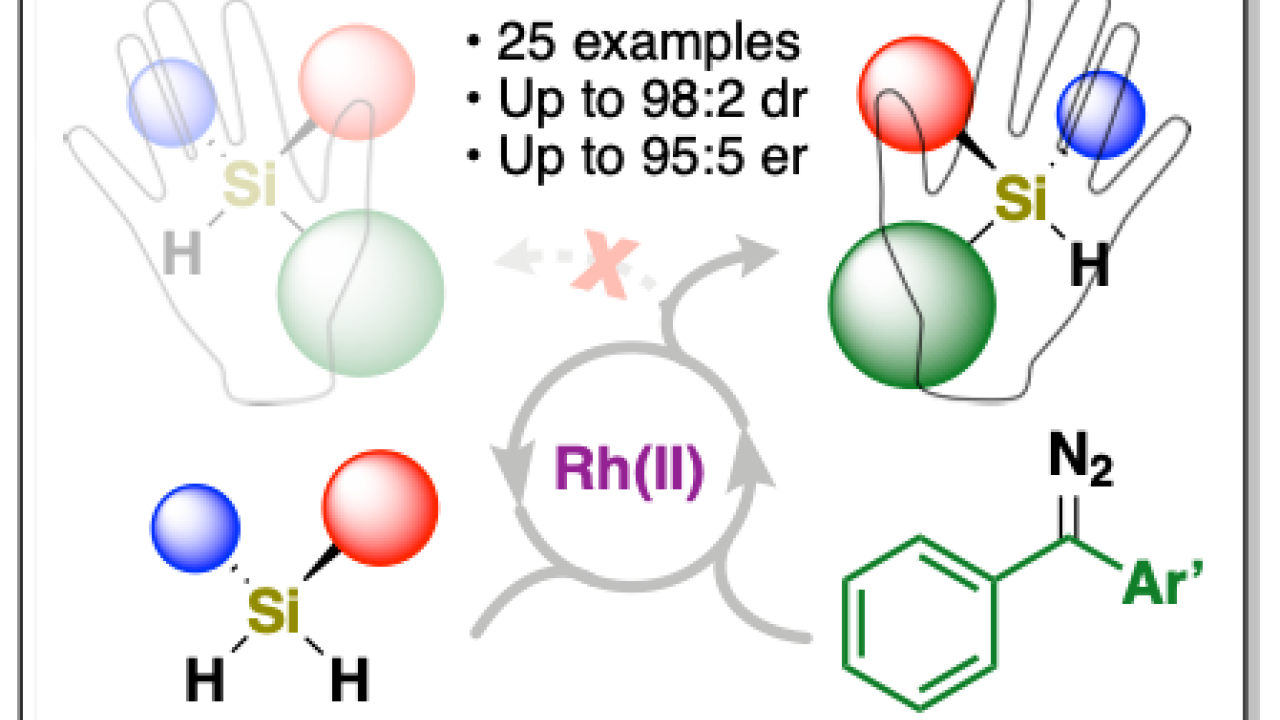
In a recent publication (Jagannathan and coworkers) in the Journal of the American Chemical Society, the Franz lab has joined forces with the Shaw lab to develop a new catalytic Si-H insertion reaction using diarylcarbenes that allows selective synthesis of chiral-at-silicon compounds with high yield and enantioselectivity. The incorporation of silicon-centered chirality into more complex structures such as drug candidates, polymers, and ligands is limited because there is a shortage of synthetic methods to access these chiral molecules. Chiral-at-silicon compounds are rare in number and there are few methods known to synthesize compounds capable of further synthetic transformations. In this paper, a study of 25 examples varying steric and electronic effects of reagents allowed a new synthetic method to be developed using a structural motif that increases enantioselectivity and yield of products. The results of this work expands the library of chiral-at-silicon compounds, provide mechanistic details into carbene chemistry, and demonstrate further synthetic utility of products derived from this method.
More information is available at: https://pubs.acs.org/doi/abs/10.1021/jacs.0c04533
